Reports + Research
Parkinson’s Patients Prepare for Breakthrough Stem-Cell Derived Treatment
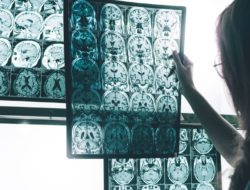
Currently under review at the FDA, a clinical trial may start next year for a novel treatment derived from stem cells that could reverse or halt the progression of Parkinson’s disease. If approved, patients in the Parkinson’s stem cell trial would first have a skin biopsy. Cells from the biopsy would be transformed “back in time” to stem cells and then redifferentiated into the brain cells that are lost in the disease. These brain cells would then be implanted into the brain with the hope that they would integrate and stop the disease from progressing. Parkinson’s disease is the second most common degenerative neurological disorder after Alzheimer’s disease and is characterized by a progressive loss of movement and increased muscle tremors and shaking.
Molecular Assemblies Named a Top 10 Startup to Watch by C&EN
The annual Top 10 startup list from Chemical & Engineering News (C&EN) included local company, Molecular Assemblies. Molecular Assemblies has pioneered a new way to make synthetic DNA using enzymes instead of the traditional phosphoramidite chemistry, which was invented and commercialized in the 1980s. To make long strands of DNA with the traditional method, one must make very short strands and then piece them together. This new enzymatic synthesis method technically has no limit to length. It also does not use harsh chemicals, making the process greener and more efficient.
FierceBiotech Names Jennifer Carver a Fierce Woman in Life Sciences
Jennifer Carver started as employee number seven at La Jolla Pharmaceuticals, and the company has had more than 300 employees at its high point, reports FierceBiotech. She started in clinical development and now is chief operating officer. Over the past five years, Carver has seen three therapies from development to commercialization. The most recent drug to receive FDA approval is Giapreza. which increases blood pressure in adults with septic or other distributive shock.
Scripps Research and Janssen Publish on Universal Flu Vaccine from Llama Antibodies
Published this week in Science, a team of international researchers led by Scripps Research with participation by Janssen scientists transformed llama antibodies into a four-in-one mega protein capable of neutralizing 59 different strains of influenza A and B. Delivered as a nasal spray, this treatment, currently in preclinical development, could provide widespread and potent protection during flu season.
Read + Listen
POW! Podcast of the Week
Omics Omics blogger, Keith Robison, and the Chief Science Officer at sequencing marketplace, AllSeq, Shawn Baker, discuss the Illumina acquisition of Pacific Biosciences for $1.2 billion.
Events
It’s Happening Here
November 14:
CLSA: 2019 California Life Sciences Industry Report Launch will be made public through a webinar on November 14
November 15:
BIOCOM’s annual Celebration of Life dinner will take place the evening of November 15
WIB-Southern California Presents: Cannabinoid Research and Orphan Diseases on the evening of November 15
Client disclosure: Molecular Assemblies, Scripps Research
Tags: Biotech, Biotech Briefing, Life Sciences, Medical Research, Startups